 Cake, the secret weapon to creating a superconductor that can work at room temperature. Actually, to be more precise, a layer cake. Now don't get your knickers in a bunch, I have not finally jumped off the deep end, the layer cake method is what scientists have used in order to achieve almost every one of the Type II superconductors. The discovery and usage of this method was a triumph, they made a note "huge success". It is hard to overstate their satisfaction when they figured out that by using cake, they could get a superconductor at 35 degrees Celsius. Let's take an example superconductor, named D223. This superconductor is a silicon and copper superconductor. (As you recall, copper by itself cannot create a superconductor due to the format of its unit cell. However, in conjunction with other elements it can.)
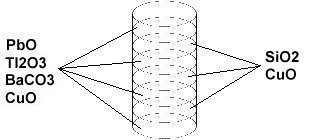 Here is a basic visual representation of the layering method for D223. To form this superconductor, a "pellet" of silicon dioxide and copper oxide and a pellet of lead oxide, dititanium trioxide, barium carbon trioxide, and copper oxide are created. Each pellet is just a layer with a specific stoichiometric ratio between the different molecules. The two types of pellets are then layered on top of each other, like a cake, with intermediary spaces between them, like frosting.
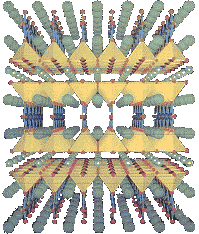 This is a more detailed version of a Type II superconductor with the layers of different elements. So, as opposed to the random assortment of atoms in a Type I superconductor, the layer cake method allows for not only higher temperature superconductors, it makes it easier to measure because of the more controlled creation. Finally, this method of creation allows for scientists to see what is working and tweak it to make a much better superconductor. If you want to know more about the importance of the crystal structure and high-temperature superconductors, click here.
 What if we could grow wires on trees? A crazy idea, I know, but it is a surprisingly plausible one. Organic compounds have shown surprising promise as conductors of electricity.
These organic molecules can be found in nature, and, do I dare say, chemists and physicists are now working together to study this neat combination of fields. At Clark University, chemists are altering the electron densities and bond lengths for materials, which are then studied for superconductive effects at low temperatures.
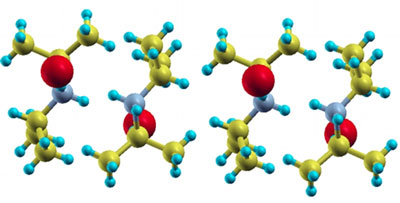
One example, diisopropylammonium bromide (DIPAB), is a ferroelectric compound (image above) that shows promise. When electricity is passed through the superconductor, electric dipoles in the material line up. In this way, the inter-molecular forces within the material align themselves for conducting moving electrons.
Just imagine, one day we may be growing out circuits on trees! If this isn't amazing, I don't know what is.
 Type one elements are all somewhat conductive at room temperature. It would stand to reason then that all elemental conductors can become superconductors, but this is not the case. Gold, silver and copper, the three best room temperature conductors cannot become superconductors. All three elements have only one valence electron, which makes it easy to carry a current, so what's preventing them from getting to superconductivity? One simple word, structure. The structure of their unit cell is a face centered cube (FCC).
So what, you may say. I've seen a list of superconductors, and lead, which also has a FCC is able to become a superconductor (at 7 K).
Well, the fact that it's unit cell is a FCC is important but that is not the only limiting factor. Let me explain. A FCC has the most atoms per cell of the basic unit cells, 4. This makes them very tightly packed, leaving very little room for movement. Superconductors require flexibility within the lattice in order for the cooper pairs, which are a pair of electrons, to form (called the electron-phonon effect). The FCC cannot vibrate enough for the pairs to be created, a dampening of the electron-phonon effect.
But, then why is lead and other FCC's able to become superconductors? They can form because their modulus of elasticity is high, making them much more flexible. This prevents the electron-phonon effect from being dampened.
 As you know by now this blog is all about superconductors, so I bet you're wondering, why is this post about the different types of diabetes Well, this post is not, in fact, a post on diabetes but about two different types of superconducting materials. Type one superconductors were the first type to be discovered and have a few quirky traits.
They are mostly elements and have "perfect" diamagnetism. When they enter the superconductive state, the resistance suddenly drops to zero, as shown by the picture above. Type ones are mainly metals and metaloids and form only at very, very (7 K) low temperatures. From now on we'll refer to these types of superconductors as "soft" ones, while type two will be called "hard". To access a list of soft superconductors, click here.
Hard superconductors are almost 100% man-made alloys and metaloid compounds. They were first created in 1930 but not recognized until after the discovery of the Meisner effect. These superconductors are created using the layer cake method. This makes these types of superconductors more stable at higher temperature, allowing them to be nicknamed "high temperature" superconductors (30 degrees Celsius). Hard superconductors are broken up into more specific categories based on the ratio of the elements, such as the perovskites. Due to the hard superconductors stability at high temperatures, they are much better for industrial use (Maglev trains, MRI machines) than soft ones.
Some believe that there will be a limit on the temperature that hard superconductors will be able function but as of right now we have not found such a limit. Personally, I believe that as the technology we create improves, so will the top temperature and the usability of these superconductors.
 Ever heard of levitation? You know, that floating trick that you see in Star Wars or those other science fiction pieces? Well, guess what. It's no longer science fiction. At a TED Talk, Boaz Almog shows how a 3-inch thick disk can levitate over a magnet. Even more stunning, he explains that a similar disk could be used to carry a small car on top, while levitating above the ground. How? Through a process called quantum locking.
The disk in the video (click the picture to watch the TED Talk) has a superconductor layer about half a micron thick. To put that into perspective, if 2,000 identical layers were put on top of each other, that would just barely make a millimeter. Anyways, that disk, hardly 3 inches thick, can flip, spin, and slide in midair through a process called "quantum locking", a result of the Meissner effect. I'll try to summarize it as best I can, but you really need to look at the video, quantum locking is pretty amazing.
Superconductors are, well, super good at conducting electrons. In the face of a magnetic field, this causes the surface of the superconductor to form currents, which nullify the magnetic field. To look at it another way, there is absolutely no way for a magnet to force a magnetic field into a superconductor. This is, in essence, the Meissner effect: the superconductor resists any magnetic field, so it is repelled by the force of the field.
Now, in a perfect superconductor, this would essentially be the same as pointing the same sides of two magnets together: they would push in opposite directions. This is far from stable, and thus a perfect superconductor is not perfect for levitation. Rather, the keys to levitation are the superconductors which aren't perfect. Super-thin superconductors, for instance, have small "weak spots" where the field from a magnet can just barely pierce through, forming something called a flux tube. When these flux tubes form, they essentially anchor the superconductor into its space. This is why you can tilt a levitating superconductor and it will remain motionless in midair.
Now let me ask you: what can't superconductors do?
 Superconductivity, as defined by Merriam-Webster, is a complete disappearance of electrical resistance in a substance especially at very low temperatures. That is a very basic definition that is not completely accurate (which will be explained later). But, then what is a superconductor? In order to create a better, more detailed definition, the characteristics of superconducting materials should be understood. Superconductors are characterized by a repulsion of magnetic fields, which was discovered by W. Meissner and R. Ochsenfeld in 1933. This discovery occurred 22 years after the initial discovery of superconductors in 1911 by H. K. Onnes, when he observed a second characteristic of superconductors, a complete lack of electrical resistance. For a more detailed history of superconductors and their discoverers, click here.
As you've probably seen, the first definition did not make mention of the repulsion of magnetic fields, called the Meissner effect, but that's not why that definition is flawed, as this is a result of the lack of resistance. Rather it is the second part, the very low temperature part, which is wrong. As scientists are able to synthesize superconductors, higher temperature ones have been created. High being relative, since the temperature are still cold, but they are much higher than the 4 K Mercury that Onnes discovered superconductivity with.
So, superconductors, what are they? They are materials, usually metals, that when brought down below a certain temperature experiences a complete lack of resistance. This definition (and brief history lesson) should make understanding superconductivity a bit easier.
|








 RSS Feed
RSS Feed