"it is this interaction that leads to the electron pairing—the slight attraction between electrons—that in turn results in superconductivity" - Tahir-Kheli, Senior Staff Scientist at the California Institute of Technology
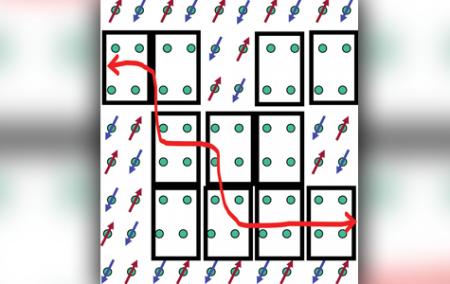 One of our first posts talked about water doping. At the end of the 2012, doping came up again in a post about the pseudogap. Well, who would have guessed it, doping has come to superconductivity once again. Most of the "high-temperature" superconductors started out as magnetic insulators that were "doped", artificially removing electrons for the original material, consequently changing the molecular structure of the original material. The result, if done correctly, is a superconducting cuprate material. But, why? Why does doping make things become superconductive? Well, a team at the California Institute of Technology have spent 5 years trying to figure that out, and the team thinks they may know. They found out that, when strontium atoms were used to replace lanthanum atoms and subsequently remove electrons from a cuprate, they were left with a superconductor. A superconductor which, when looked at on the molecular level, had formed four-centered "holes" of copper atoms aligned around the doped strontium atoms. Apparently the electrons within these "holes" (referred to the team as "plaquettes") exhibit metal-like behaivor, while the electrons outside the plaquettes acted as magnetized insulators (see image). Electrons travel freely through the plaquettes and experience slight attractive forces from the magnetized electrons. According to this paper, these plaquettes must be close together in order to trigger superconductivity, and the isolation of plaquettes may manifest itself as the pseudogap. Bear in mind that this is just one theory. However, it appears to be backed by the interesting research of this team. Definitely something worth keeping in mind.
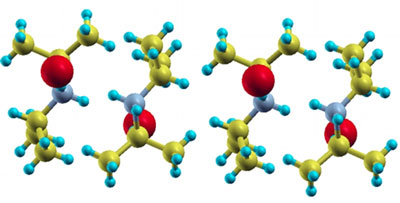 What if we could grow wires on trees? A crazy idea, I know, but it is a surprisingly plausible one. Organic compounds have shown surprising promise as conductors of electricity.
These organic molecules can be found in nature, and, do I dare say, chemists and physicists are now working together to study this neat combination of fields. At Clark University, chemists are altering the electron densities and bond lengths for materials, which are then studied for superconductive effects at low temperatures.
One example, diisopropylammonium bromide (DIPAB), is a ferroelectric compound (image above) that shows promise. When electricity is passed through the superconductor, electric dipoles in the material line up. In this way, the inter-molecular forces within the material align themselves for conducting moving electrons.
Just imagine, one day we may be growing out circuits on trees! If this isn't amazing, I don't know what is.
 Type II superconductors were first introduced in a Russian journal in 1954. Since then, Nobel prizes have been awarded for people who contributed to its understanding. For the past ~60 years, people have generally understood that magnetic fields penetrate type II superconductors. When placed in a magnetic field, the superconductor becomes pierced with small "vortexes" of electric current arranged in a regular lattice pattern (see image).
However, in 2008, a group led by Notre Dame physicist Morten Ring Eskildsen made a discovery that may alter our understanding of Type II superconductors. This group studied the "vortex lattice" formed in a certain superconductor made of cerium, cobalt, and indium (CeCoIn5) as the magnetic field strength was increased.
The general expectation is that an increased magnetic field will increase the presence of current "vortexes" through the superconductor, thereby disrupting the superconductive nature of the material. However, according to Eskildsen's team, the magnitude of penetrating currents through the material actually decreased. This new research significantly complicates our understanding of how Type II superconductors react to magnetic fields.
One answer may be the unit cell structure of these superconductors. Simply by looking at it intuitively, it is easy to think that the way that the atoms of the material are aligned may have something to do with directing the current "vortexes". Really, at this point, there is no clear answer. However, these findings by Notre Dame may very well alter our understanding of Type II superconductors.
 Ever wondered why materials need to be so cold in order to become superconductive? This is because superconductivity is believed to require the forming of Cooper pairs, which are pairs of electrons that move through the superconductive material with equal but opposite spin and angular momentum (page 86). These pairs exist throughout superconductors in tightly-knit nets with other pairs.
Now, normally, electrons have too much energy to settle into Cooper pairs (thanks to heat from the surroundings). However, something that's been noticed is that, when materials cool down to near-absolute zero, Cooper pairs form easily (too complicated? read this).
When this happens, almost all the electrons settle down to the Fermi Level of the material. What's interesting about this is that, in essence, the fermi level is the absolute minimum level of potential energy that valence electrons can hold. Think about the energy levels electrons maneuver in order for atoms to emit photons. Now think lower.
The current theory holds that superconductors are only superconductive when they are cold enough for the materials' valence electrons reach the fermi level. Manipulating that, this means that superconductors cannot form at higher temperatures, since the higher temperature will excite the electrons beyond the fermi energy levels.
However, we have clearly seen evidence of room temperature superconductivity. Am I the only one who smells a contradiction? How can anything at room temperature have electrons at the fermi level?
Right now, scientists are only testing for the prized ability of superconductors: to be superconductive. However, it seems that conventional superconductor theories are in for a little twisting and reshaping once scientists manage to fully study fermi levels of higher-temp superconductors.
"We do not claim yet a local correlation between the pseudogap and superconductivity. We don't have experimental evidence strong enough to prove such a correlation. But establishing this connection will be an important direction of future study."
Read more at: http://phys.org/news/2012-11-atomic-resolution-images-fresh-insights-mysterious.html#jCp
 For anyone well-acquainted with the idea of "high-temp" superconductors, cuprate compounds quickly come to mind. As of yet, cuprate superconductors are able to exist at relatively high temps (-100 degrees Celsius or below, yikes!). Of course, research is being done about possible superconductors that could work at, say, room temperature. That's where the pseudogap comes into play. As mentioned in the linked article, the pseudogap phase is a state of non-superconducting behavior found in superconductors (mostly cuprate) near the point when the superconductor should be in a proper environment for superconductivity.
The interesting part of the pseudogap phase is what was found by this international research team. The researchers noticed that, when studying samples (which become superconductors after sufficient doping) under a scanning tunneling microscope, the sample underwent interesting physical changes just as the superconductor moved from the pseudogap state to superconductivity. The researchers saw various nano-scale clusters of atoms accumulate as doping brought the sample closer and closer to the point of superconductivity. As the samples transitioned from pseudogap state to superconductivity, the researchers saw these clusters of atoms start to join together.
What does this remind you of? For me, it reminds me about the unit cells of crystalline solids. Crystalline solids form the unique shapes that they do because, on the atomic level, atoms or molecules line up into lattice structures, with the smallest repeated pattern called the unit cell.
Now, when the sample compounds reached superconductivity, the researchers saw the "clusters" of atoms fully connect with one another. A possible thought is that these clusters, when fully connected, form a lattice similar to that of crystalline solids. For crystalline solids, this kind of lattice led to rigid shapes in the macroscopic world. What if, for superconductors, the atoms form a similar lattice that allows for superconductivity? I am not saying that the lattice would need to be identical to those of crystalline solids, but I think it is reasonable to believe that they could be similar in concept. Frustratingly enough, this can all only be speculation, since very little is actually known about the pseudogap and superconductivity.
 Ever heard of levitation? You know, that floating trick that you see in Star Wars or those other science fiction pieces? Well, guess what. It's no longer science fiction. At a TED Talk, Boaz Almog shows how a 3-inch thick disk can levitate over a magnet. Even more stunning, he explains that a similar disk could be used to carry a small car on top, while levitating above the ground. How? Through a process called quantum locking.
The disk in the video (click the picture to watch the TED Talk) has a superconductor layer about half a micron thick. To put that into perspective, if 2,000 identical layers were put on top of each other, that would just barely make a millimeter. Anyways, that disk, hardly 3 inches thick, can flip, spin, and slide in midair through a process called "quantum locking", a result of the Meissner effect. I'll try to summarize it as best I can, but you really need to look at the video, quantum locking is pretty amazing.
Superconductors are, well, super good at conducting electrons. In the face of a magnetic field, this causes the surface of the superconductor to form currents, which nullify the magnetic field. To look at it another way, there is absolutely no way for a magnet to force a magnetic field into a superconductor. This is, in essence, the Meissner effect: the superconductor resists any magnetic field, so it is repelled by the force of the field.
Now, in a perfect superconductor, this would essentially be the same as pointing the same sides of two magnets together: they would push in opposite directions. This is far from stable, and thus a perfect superconductor is not perfect for levitation. Rather, the keys to levitation are the superconductors which aren't perfect. Super-thin superconductors, for instance, have small "weak spots" where the field from a magnet can just barely pierce through, forming something called a flux tube. When these flux tubes form, they essentially anchor the superconductor into its space. This is why you can tilt a levitating superconductor and it will remain motionless in midair.
Now let me ask you: what can't superconductors do?
|






 RSS Feed
RSS Feed